
Priority is often given to Priestley because his work was published first. The chemists used a scale such that the natural mixture of oxygen isotopes had an atomic mass 16, while the physicists assigned the same number 16 to the atomic.It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen was isolated by Michael Sendivogius before 1604, but it is commonly believed that the element was discovered independently by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774. Atomic mass is based on a relative scale and the mass of 12C. Oxygen is the chemical element with the symbol O and atomic number 8.So, when most of us refer to oxygen it is the oxygen with 8 neutrons. Structure Of Oxygen Oxygen is a separate element, which has a chemical formula of just one oxygen atom. 58.44 g per mole is the result of combining these two masses.

That being said, the most common isotope is the first one. The atomic mass of chlorine is 35.45 g per mole, while the atomic mass of sodium is 22.99 g per mole. However, this atomic mass is the average atomic mass of all the isotopes in nature. Oxygen makes up almost half of the Earth's crust in the form of oxides. The average atomic mass of oxygen is 15.9994 atomic mass units.At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O2.ĭiatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time.
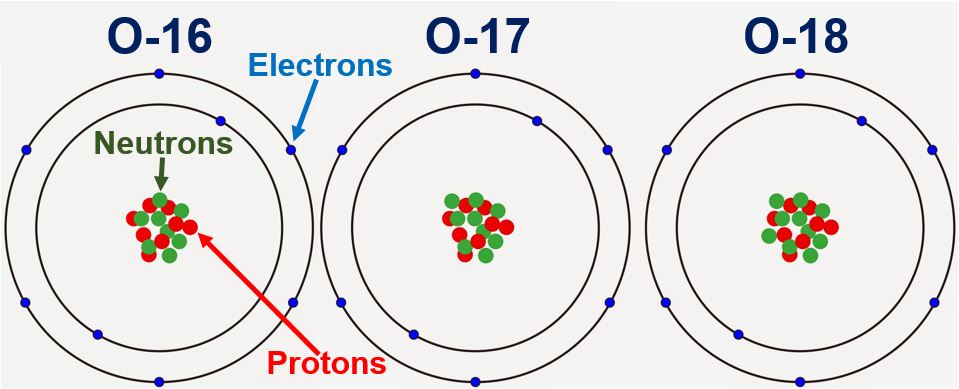
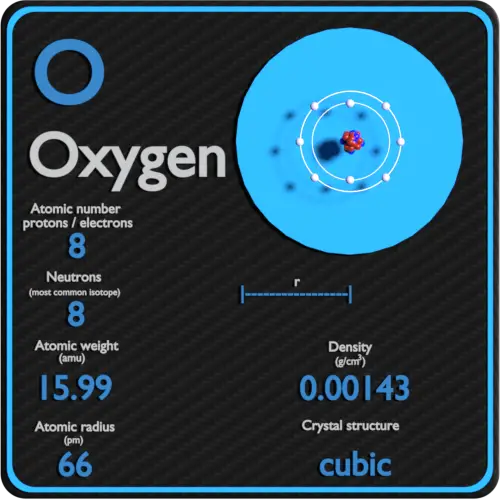
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to. However, this atomic mass is the average atomic mass of all the isotopes in nature. One molecule of water (H 2 O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.The average atomic mass of oxygen is 15.9994(16) atomic mass units. The average atomic mass of oxygen is 15.9994 atomic mass units.


 0 kommentar(er)
0 kommentar(er)
